TLC
TLC
Thin Layer Chromatography: How To
What is TLC?
Thin Layer Chromatography (TLC) is a commonly used analytical technique that allows for rapid and inexpensive analysis of various mixtures. For organic chemists, TLC is most commonly thought of as being done on silica plates. In reality, many more sorbents in TLC format are available: aluminum, C18 reverse phase, cellulose, ion exchange resins, and many more.
Besides speed and low price, there are three more features that make TLC very popular:
- It is easy to run up to 20 samples simultaneously
- It is possible to use a square plate, run in one direction with one elutant; then rotate plate 90deg and run with another elutant (creating a "two-dimensional" TLC). Besides providing better resolution, this experiment determines whether any of the mixture components happen to be unstable under separation conditions.
- Plates (unlike HPLC columns) are disposable.
Setting up a TLC analysis is very simple. A suitable beaker or a jar with some kind of cover, a piece of filter paper, glass capillaries, and the TLC plate itself constitute all the materials needed. TLC plates differ on backing material. Aluminum, polyester, and glass are the most common materials. Some people insist on glass backing, but, in our experience, aluminum seems the most convenient.
Setting up and running a TLC
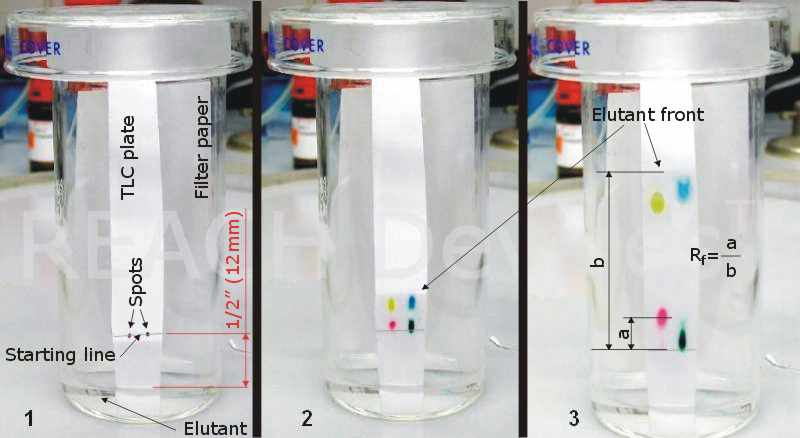
A TLC plate must be cut/divided to size (most of them come as 20cm x 20cm or 20cm x 5cm sheets) and a start line drawn lightly with soft pencil (no inks!) at about 3/4" (17mm) from the bottom edge. The solution of material to be analyzed is carefully spotted onto the start line (this step is discussed in greater detail in the next section).
A small amount of elutant is poured into jar (to a depth about 1/2 to 2/3 of the bottom of TLC sheet-to-start line distance), and a rectangular piece of filter paper inserted so that it touches the elutant and lies on the jar wall, mostly above the elutant pool. The top edge of filter paper should be close to the jar mouth. A cover is placed on top of the jar, and the jar is tilted temporarily so that all the filter paper gets soaked in elutant. This step ensures that all inner volume of the jar is saturated with elutant vapors.
Then, the spotted TLC plate is inserted carefully into the jar, replacing the lid afterwards. The elutant must not touch the spots. It is a good practice to allow for 1/2" (12mm) between elutant level and spots. The picture to the left shows what happens next. Here, felt pen inks were subjected to the analysis, so results are immediately seen (are colorful and visible to the naked eye). More often, however, the spots are colorless, so some kind of visualization is required.
After the solvent front reaches within ~1" (25mm) of the top edge of the TLC plate, the plate is removed from chamber, and the exact elutant front location is marked with a pencil. This mark is needed to calculate Rf (Retention factor) values.
Spotting a TLC
To spot a TLC, first dissolve the material to be separated in a solvent. Then, draw some solvent into a capillary, and press the capillary onto the TLC place medium. If done correctly, the solvent should all drain onto the medium, creating a wet circular spot. The solvent is then allowed to evaporate, leaving behind only material to be separated, before placing the TLC plate into the elutant to "develop".
Spotting a TLC plate does require some practice. Three common mistakes, which always lead to overloading the TLC plate, are very popular. Due to these, instead of neat spots large streaks appear, which bear no information.
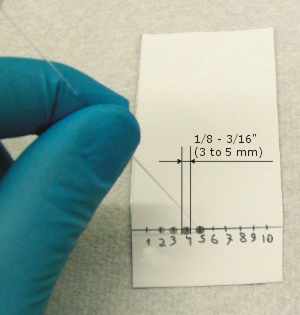
- Spotting too much of a solution. The capillary diameter should be around 1/32" (0.7 to 1mm). The initial spot diameter should be 1/8" to 3/16" (3 - 5mm) immediately after application.
- Spotting too concentrated or non-homogenous of a solution. The solution to be spotted should be free of precipitate and insoluble oils, and should not be too concentrated. 0.5 to 5wt% is good enough. The right dilution is found by trial and error.
- A not too volatile solvent is used. There is no point in spotting using solutions prepared in DMF, Dimethylacetamide, DMSO or pyridine, to name a few. The spotting solvent must be completely removed from the TLC before developing. For DMF and pyridine, it is possible to place the spotted TLC plate for ~5min into vacuum before the run to evaporate the solvent. For DMSO and Dimethylacetamide, vacuum won't work - one has to do a mini workup to remove these solvents first.
If a solution to be spotted happens to be too diluted (like after a column when a too low of Rf fraction was finally pushed out with large amount of elutant), then it is possible to spot same place a few times. Just allow the solvent to dry between spottings. This, however, leads to poorer TLC resolution.
Spotting capillaries are available commercially. Do not use melting point determination capillaries - they are way too thick. It is also possible to pull a capillary out of a pipette with a good gas burner.
How to choose a solvent (elutant) for TLC
Choosing a right elutant (or elutant mixture) to obtain an useful TLC requires some trial and error. Usually a TLC is informative enough if the lower spot has Rf > 0.1 while the top spot exhibits Rf < 0.85.
For a silica coated TLC plate, the elutant 'strength' ie ability to drag a spot up the plate can be approximately represented by the following sequence (in order of increasing strength):
Perfluoroalkanes (weakest), Hexanes, Pentane, Carbon tetrachloride, Benzene/Toluene, Dichloromethane, Diethyl ether, Ethylacetate, Acetonitrile, Acetone, 2-Propanol/n-Butanol, Water, Methanol, Triethylamine, Acetic acid, Formic acid (strongest)
So basically one needs to use stronger elutant if spots are too low or weaker elutant if spots are too high. Often it is more convenient to use the same mixture of elutants while changing their ratio to adjust the total strength of a mixture. For this approach to work one component of the mixture must be a weak elutant while another must be of excessive strength. For instance ethylacetate : hexane mixture is very popular for eluting TLC of medium polar materials. So if say 5:95 ethylacetate : hexane mixture would barely move spots, then try 10:90 or 25:75 mixtures in order to increase Rf values of the spots.
There is one more subtle thing about elutant mixtures. It is sometimes possible to separate overlapping spots while trying different mixtures of similar strength. For example if 30 : 70 ethylacetate : hexane produces Rf1 = 0.30 and Rf2=0.30 (which means spots are overlapping) for a some mixture then 5 : 95 methanol : dichloromethane or 3 : 97 acetone : toluene mixture likely to produce Rf values in the similar range (0.3 to 0.4) but it well might happen that Rf1 will become different to Rf2.
Visualizing a TLC
If compounds are colored the separation results are immediately observable. However, most substances are colorless, so use some of visualization techniques are usually required.
REACH Devices, LLC.
6525 Gunpark Drive, Suite 370-179, Boulder, CO 80301
Call: (720) 288-5722
Inquiries: support@reachdevices.com
Ⓒ 2010-2025 REACH Devices, LLC. All rights reserved.