TLC STAINS
TLC STAINS
Thin Layer Chromatography stains
TLC Stains
For colorless compounds, a visualizing technique is needed to observe TLC results. Stains can be applied by spraying or by dipping of a plate into solution. The latter is by far more convenient. However, in order to work, the right the stain solution SHOULD NOT dissolve analyte spots. For example, permangantate stain is perfect for most not-too-polar organic compounds while acetone-based nynhydrin stain is excellent for amino acids. If analyte solubility in stain solution is inevitable, try to dip the plate as quickly as possible, and then immediately wipe off an excess of stain. Still, there will be some artificial tails added to spots. Also, do not forget, if a compound that must be analyzed is volatile, it may evaporate before the stain visualizes it, especially if heating is required for visualization. The table below represents a few of these techniques:
| TLC Staining procedure | TLC Stain Recipe | Stain chemistry / physics | Comments | ||||||||
| 254nm UV light | 254nm UV lamp with filter. Darker spots on light green if plates with VU indicator are used. Occasionally brighter spots (typically blue) | UV light excites a fluorescent additive in TLC plate. Compounds screen some of the UV, making fluorescence weaker. Sometimes, visible fluorescence is exited by UV making a spot brighter, and so is colored differently than the background | A compound must have a chromophore. Even then, this technique is NOT sensitive for isolated alkenes and benzyl ethers. Fluorescence is often excited in polyconjugated compounds (benzophenone, antracene and so on). | ||||||||
| Permanganate stain |
|
Oxidative stain. Strong heating required. 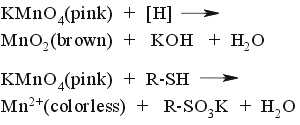 |
The most universal stain. Harsh oxidizer. Any oxidizable compound such as alcohol, ether, ester, alkene, alkyne, alkyl aromatic, ketone, carboxylic acid, amine, amide and almost all the rest will show up as brown-yellow spots (MnO2). Strongly reductive compounds such as thiols, phosphines and even dienes will show up as white spots (Mn+2) BEFORE heating. Alkanes and pyridine won't show-up at all. This stain is NOT compatible with elutants containing triethylamine - the entire plate will became yellow before spots develop... | ||||||||
| Iodine chamber | Prepare a well-closed chamber with a few crystals of elemental iodine. Let it sit for 30 min to equilibrate. Put a TLC plate inside, keep the plate for 2 sec to 10 min inside (trial and error) and then take the plate out. Watch the color change. Pick the best moment to circle spots - they might soon disappear. | Different absorption of brown colored iodine on clean silica as opposed to silica loaded with analytes. Spots may be darker or lighter than background. Thiols will oxidize to disulfides: 2R-SH + I2 = R-S-S-R + 2HI |
Hit and miss stain. Works in about 50% of all cases. Works with alkanes! Thiols and phosphines will immediately show up as white spots. | ||||||||
| PMA stain (Phosphomolybdic acid stain) |
|
Oxidative stain. Strong heating required. H3[P(Mo3O10)4] * xH2O is Mo6+ compound, light yellow. Upon exposure to reducing organics Mo5+ and Mo4+ compounds are formed (molybdenum blue, so called Keggin structure) which are green or blue colored |
Expensive. Fairly universal stain. Some amines, amides and oxidation-resistant aromatics won't show up. Stain solution is somewhat light-sensitive. | ||||||||
| CAM stain (Hanessian's Stain; Cerium Ammonium Molybdate stain) |
|
Oxidative stain. Strong heating required. Procedure same as above |
Very universal stain. More sensitive than PMA stain (above). | ||||||||
| p-Anisaldehyde Stain |
|
Some heating required. Chemistry of this stain is unknown. Probably something along the following lines: 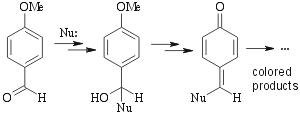 |
Not as universal as oxidative stains. Works for allylic alcohols (green spots), phenols (violet spots) amines, aldehydes, ketones, carbohydrates, esters like alkylphtalates (blue/red spots) and some other compounds. Alkenes, alkynes and aromatic compounds will NOT show up. | ||||||||
| Ninhydrin Stain |
|
Gentle warming required. Blue-pink color is due to the formation of Ruhemann's purple: 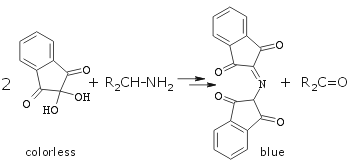 |
Specific stain for amino acids (see group TLC below) and primary amines. Secondary amines stains light yellow which is difficult to see. Tertiary amines do not stain.(An interesting review:Friedman, M. J. Agric. Food Chem. 2004, 52, 385-406.) See ninhydrin stain colors for all aminoacids! |
REACH Devices, LLC.
6525 Gunpark Drive, Suite 370-179, Boulder, CO 80301
Call: (720) 288-5722
Inquiries: support@reachdevices.com
Ⓒ 2010-2025 REACH Devices, LLC. All rights reserved.